
Dec 1, 5:53pm: Will a device from Neuralink receive FDA approval for implantation in a human? → Will a device from Neuralink receive FDA approval in 2023 for implantation in a human?
🏅 Top traders
| # | Name | Total profit |
|---|---|---|
| 1 | Ṁ5,475 | |
| 2 | Ṁ3,149 | |
| 3 | Ṁ673 | |
| 4 | Ṁ287 | |
| 5 | Ṁ229 |
People are also trading
@JanLukasR I also didn‘t expect this much traction and would have thought more carefully about how to phrase the question, all criticism in this regard is very valid.
@JanLukasR That's what I said, but I was forced to sell because I thought admins would resolve N/A. Could have made another 200 mana if I knew you weren't inactive..
@JanLukasR I think your decision to resolve it based on original intent is incorrect. If a large percentage of people in the market believed it was asking a completely different question than it was, (and their incorrect interpretation was as valid and likely as the ultimately correct one) then it doesn't meaningfully predict anything or serve any useful purpose.
@akrasiac I don’t think it is reasonable to assume this market wasn’t about the immediate first step in the process of getting this product to market. Arguing otherwise is to insist that the first human implantation that is relevant is the first commercial (non-research) instance. That makes zero sense unless explicitly stated in the criteria by the creator. The bettors should not get to decide the criteria, but they can argue their take as long as they accept they are gambling on their persuasive ability and not the market.
@BTE My alternative interpretation does not hinge on the meaning of "first" but rather on the meaning of "FDA approval."
@akrasiac And the FDA approved therm, to put their brain chip in a human. Not multiple humans, a human.
Probably going to have to resolve this N/A.
I had interpreted this the same way @akrasiac had, but clearly others didn't from trading behaviour and discussion.
Will wait a while for the creator for now. Will probably N/A it before the end of the year as otherwise people will just buy yes because either it resolves N/A or passes trials and resolves Yes (although very unlikely timeframe).
There is no way this question should be interpreted as a regulatory approval for sale. That is obvious and requires minimal research to see that a trial requires human implantation and trials that require human implantation must have the design approved before it is allowed to start enrolling patients.
@BTE The phrase "FDA approval" is always used in medicine to refer to marketing authorization. In every one of the many conversation I've ever had about drug development, when someone says that somthing has/will "receive FDA approval," they always mean marketing authorization. The FDA agrees, see here.
@akrasiac "FDA approval of a drug means that data on the drug’s effects have been reviewed by CDER, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population."
FWIW, I have very little invested here, but I think N/A is the right resolution -- when large groups of people interpret a question completely differently, the market no longer serves the intended purpose of a prediction market.
@akrasiacThis is not a drug. Do they have to APPROVE a trial design for implantation prior to trial? YES they do. So a strict reading would make both of us correct.
@akrasiac The proper language for medical device is actually "cleared". Medical devices do not receive approval like drugs do. They are different levels of scrutiny.
@BTE Nope, "approval" is also the proper language for a device, see here.
Obviously, approval has a colloquial meaning and it could certainly be used in either of these two senses. In my experience, it is generally used in the marketing authorization sense. But you have also shown instances where it's used the other way. I think we can agree that "approval" here is ambiguous, the market creator did not clarify that ambiguity.
@akrasiac You are correct, but only Class III medical devices are "approved". Class I & II are only "cleared" based on equivalency to an existing device. Almost all medical AI falls in Class II. Neuralink being an implanted device does require Class III approval which is much more rigorous.
@akrasiac Here is the destinction between Approved, Denied and Cleared. Device Approvals, Denials and Clearances | FDA
I think "approval in 2023 for implantation in a human" means it is about approval for implantation in a human, even if the more common approval discussed in FDA contexts is market approval. This seems like a strong argument for YES to me. (I'm aware I'm betting NO. I don't expect the better interpretation to win out here.)
Author never answered the previous question about whether this includes a trial and is now inactive, but I did some sleuthing and I'm pretty confident that this was not intended to include trials, given this comment https://manifold.markets/KeresaHoward/will-neuralink-conduct-human-trials#cCzt7Mi6OIP1oZfL9zO5
Edit: That comment is in a market about human trials. The comment links to this market about FDA approval, which suggests that the author thought trials do not count as FDA approval. It's definitely not super clear evidence, but at least weak evidence.
@jack Wouldn't it be best to say ANY neuralink device in ANY human, seeing as nothing was specified? Basically taking it at face value in plain english
@jack Yes, it says "a human" not "humans"
I can see YES or N/A depending on the argument, but I can't see NO here
@JoelTodd A medical device receiving FDA approval is not generally understood to mean the device is in clinical trials. See for example https://www.fda.gov/medical-devices/products-and-medical-procedures/device-approvals-denials-and-clearances which has zero mention of trials.
@jack "We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study!"
That is a pretty clear answer to a plain english interpretation of this market
@jack Also, the comment itself doesn't even mention trials, It says "Regarding FDA approval the market is here."
I don't understand why you're buying NO
Edit: The tweet literally says "Recruitment is not yet open for our clinical trial." implying there will be a TRIAL due to the approval.
@ShadowyZephyr Yeah the tweet mentions "FDA", "approval", and "human" I literally can't see any other interpretation I'm sorry
I don't feel like getting into this argument personally, and I don't really care about my stake because I predict the market will resolve N/A. I do want to avoid the all-too-common scenario where because the author isn't around to make a decision, the question just becomes "predict how the ambiguity will be settled".
I suggest polling a few people on how they interpreted the question to see if it's ambiguous.
@jack Given that everyone bet YES except for you I think most people think it's YES (including me) but I wouldn't be super upset with N/A if Neuralink's trial is not announced by 2023 only because the author is inactive
@jack He's commenting in a market about CONDUCTING human trials and pointing towards his market regarding the APPROVAL process which is slightly different
Not looking from a counter argument from you just throwing this alternative interpretation out there
@ShadowyZephyr By everyone you mean just you two, and you are excluding the previous commentors who also pointed out the ambiguity about trials months ago.
@jack If we're being pedantic it was one guy commenting on ambiguity so its more like a 2v2 right now lol
@jack That's why I posted it on discord, I thought that counted as YES, but I wanted more opinions to see if that was justified
@jack Okay, I think mods will resolve this N/A. Fine by me. I still think the YES interpretation is more likely.
@jack For what it's worth, I definitely traded under the assumption that "FDA approval" meant approval to bring to market. This interpretation is mainly informed by my reading about the approval process for COVID-19 vaccines, where for some time the vaccines were only "authorized" (EUA) and not approved.
I didn't really make the distinction between "in a human" vs "in humans" because I took "a human" to refer to a "generic human" and not "any human."
Looks like we currently have 3 commenters here who thought it included approval for clinical trials, and 3 who thought it was approval to bring to market.
I also ran this Discord poll and the results show more people thought it included clinical trials, but a lot of people who thought it was ambiguous:
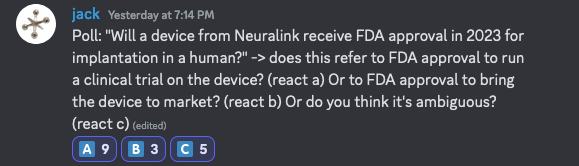
Assuming the author isn't coming back, we either need a mod to resolve, or otherwise it could resolve N/A after it closes given the ambiguity (see https://help.manifold.markets/unresolved-markets). Either way, I think N/A resolution makes the most sense.
@DavidChee I saw you commenting on the market, this will probably need mod resolution so how do you think this should be handled?
@jack Why would anyone think this is to bring it to market?!? This question is definitely about a trial.
@jack Another company recently received similar approval for a trial so it should have been clear that was the next step.
