Link: https://en.wikipedia.org/wiki/Hypothetical_types_of_biochemistry
The element silicon has been much discussed as a hypothetical alternative to carbon. Silicon is in the same group as carbon on the periodic table and, like carbon, it is tetravalent. Hypothetical alternatives to water include ammonia, which, like water, is a polar molecule, and cosmically abundant; and non-polar hydrocarbon solvents such as methane and ethane, which are known to exist in liquid form on the surface of Titan.
people with an freshman-level understanding of chemistry often posit the existence of silicon-based life, based purely on the notion that silicon is right below carbon on the periodic table and elements in the same periodic group tend to have similar reactivity, so surely if we have carbon-based life we could theoretically have silicon-based life, right?
unfortunately, this belief is specious, and deeper levels of chemical education clearly reveal obvious reasons why silicon-based life very likely does not exist -- and if it does, it is certainly not by direct analogy to terrestrial carbon-based life. to wit:
silicon does not form double bonds (in general). this is a specific application of the double-bond rule (https://en.wikipedia.org/wiki/Double_bond_rule): because silicon is a 3p element, its p orbitals are less-well suited spatially for pi bonding than carbon's 2p orbitals. an obvious example of this is the direct comparison of carbon dioxide (CO2) with silicon dioxide (SiO2); the former is a stable unsaturated diatomic molecule, while the latter forms a continuous material, an extended solid -- because silicon would rather form an infinite lattice of -O-Si-O-Si-O- bonds than commit to a molecular O=Si=O analogue to CO2.
there are exceptions to this general trend (https://en.wikipedia.org/wiki/Silenes), but they are of the type that prove the rule: silene and silyne fragments are unstable and prone to dissociation, more useful as case studies or transient synthetic waypoints for organosilicon chemists.
the ramifications of this simple fact are profound for our hypothetical silicon-based lifeform: no double bonds means no alkenes and no alkynes. no carbonyl groups means no carboxylic acids and no amides (hence no amino acids). perhaps most damning, it means no aromatic rings of any sort -- heterocyclic or otherwise. unlike carbon, silicon is simply too prone to polymerization to form the discrete molecular building blocks of life that we know and love.
@pyrylium Thanks for the comment!
A small note: you expressed SiO2 structure as -O-Si-O-Si-O-, which confused we for a moment, because that way it would clearly be SiO, not SiO2. The actual structure is more complicated (picture from Wikipedia):
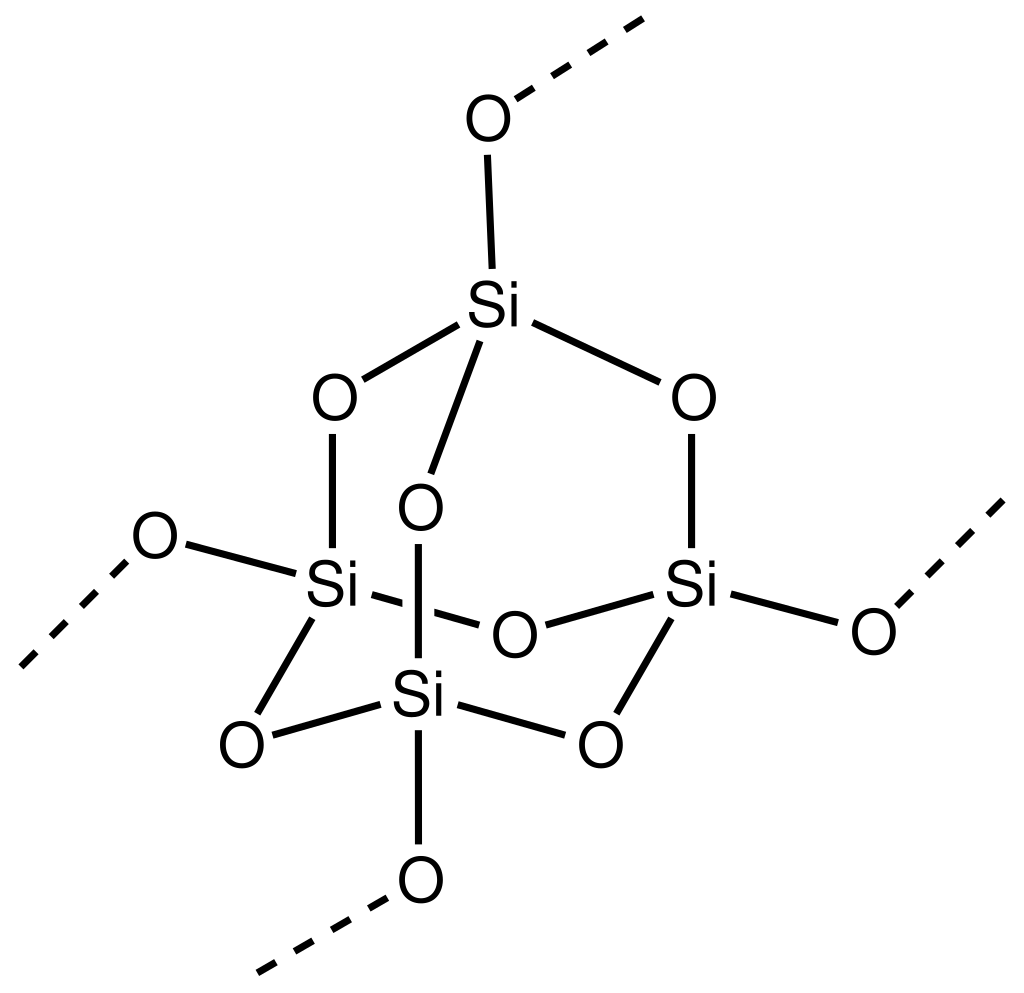
(I am writing this for others who might think of that, not because I think you don't know it)