
Definitions and Criteria [please read carefully]:
1. "AlphaFold-like system"
An AI system that uses deep learning to predict protein structures or design novel proteins with accuracy comparable to or exceeding AlphaFold 2. This includes AlphaProteo or similar tools from any company, not just DeepMind.
2. Significant Contribution:
The AI system must have played a crucial role in the drug's design, as evidenced by at least one of the following:
a) Explicit confirmation in a paper (whether peer-reviewed or published as a pre-print).
b) Official statement from the drug developer citing the AI system's importance.
c) Patent filing citing the AI tool as critical to the drug's development.
3. Passing Phase I Trials:
The drug must complete Phase I trials in humans with results indicating acceptable safety and tolerability, as reported by the drug developer or regulatory authority.
4. Commercial Potential:
The drug is likely to have significant commercial value if it's eventually approved by medical authorities. Significant commercial value is defined as at least $1 billion in projected lifetime revenue. This number would be estimated based off the company's own projections, or based off the lifetime revenue of previous generation drugs in the same category.
5. Eligible Jurisdictions:
Phase I trials must be conducted and completed in one or more of the following jurisdictions:
- United States or Canada
- Any EU Member, UK, Norway, Switzerland or Iceland
- Australia or New Zealand
- Japan
Other jurisdictions will only be accepted if there's widespread consensus among Western scientists of their Phase I trial being trustworthy.
6. Public Disclosure:
Information confirming all the above criteria must be publicly available through official sources (regulatory filings, company announcements, or scientific publications) by January 31, 2026.
Market Details:
- Betting closes: Dec 31, 2025
- Resolution date: Jan 31, 2026 (to account for 2025 data being published with a delay)
Resolution Criteria:
Yes: There is clear, publicly available evidence that a drug meeting ALL of the above criteria has passed Phase I trials, with the last day of the trial occurring no later than December 31, 2025.
No: No clear, publicly available evidence of any drug meeting ALL of the above criteria, or if no such drug has completed Phase I trials by December 31, 2025.
Question was inspired by this EY tweet:
🏅 Top traders
| # | Trader | Total profit |
|---|---|---|
| 1 | Ṁ981 | |
| 2 | Ṁ878 | |
| 3 | Ṁ622 | |
| 4 | Ṁ583 | |
| 5 | Ṁ435 |
People are also trading
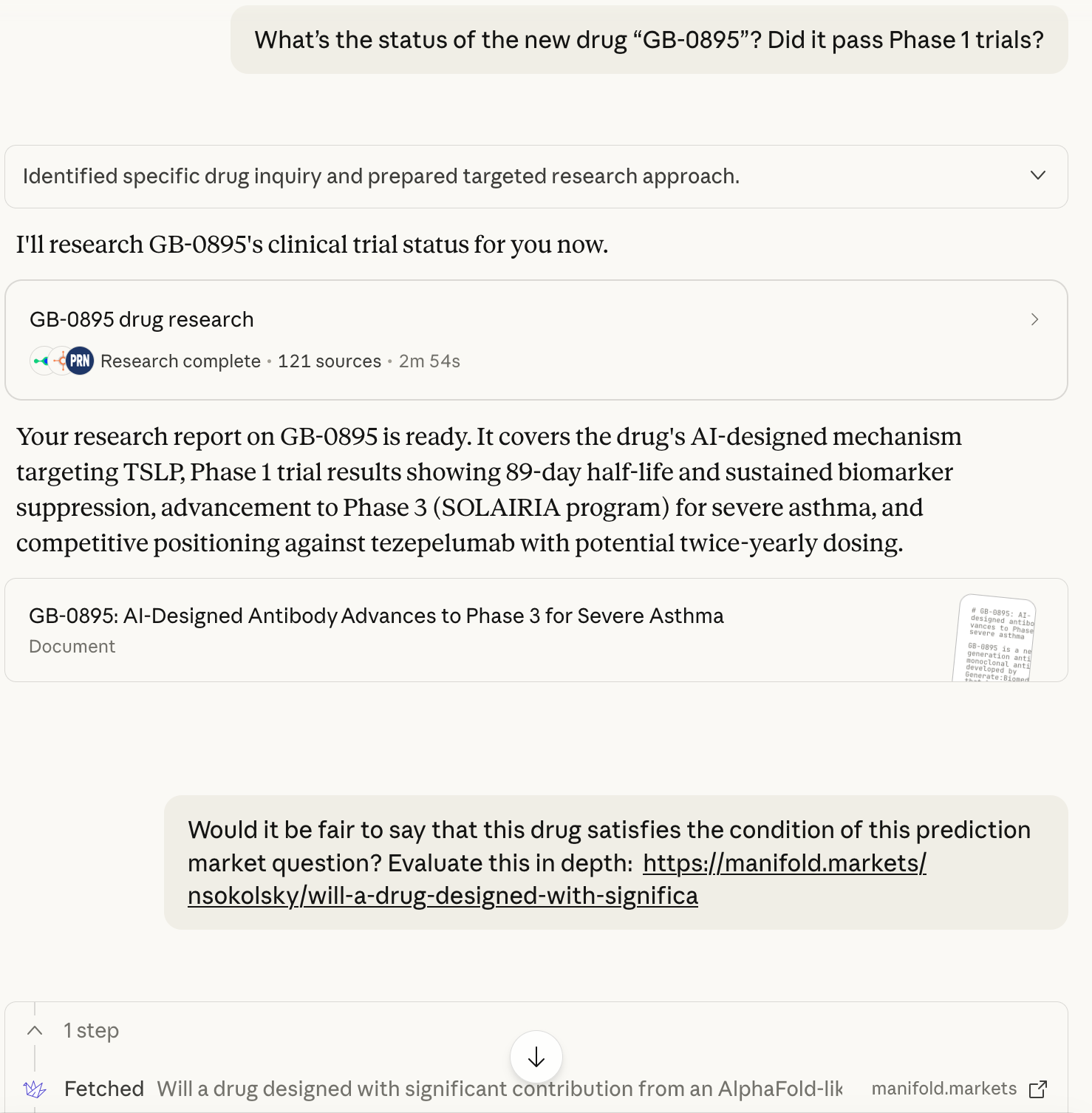
Resolving to yes due to "GB-0895" (potential asthma drug). Reasoning:
The Phase III trial has been published on the FDA website: https://clinicaltrials.gov/study/NCT07276724
Their press release is extremely confident: https://www.prnewswire.com/news-releases/generatebiomedicines-to-present-phase-1-results-for-ai-engineered-gb-0895-in-asthma-at-the-european-respiratory-society-international-congress-302566586.html
The announcement was made in late September 2025, without any negative updates since
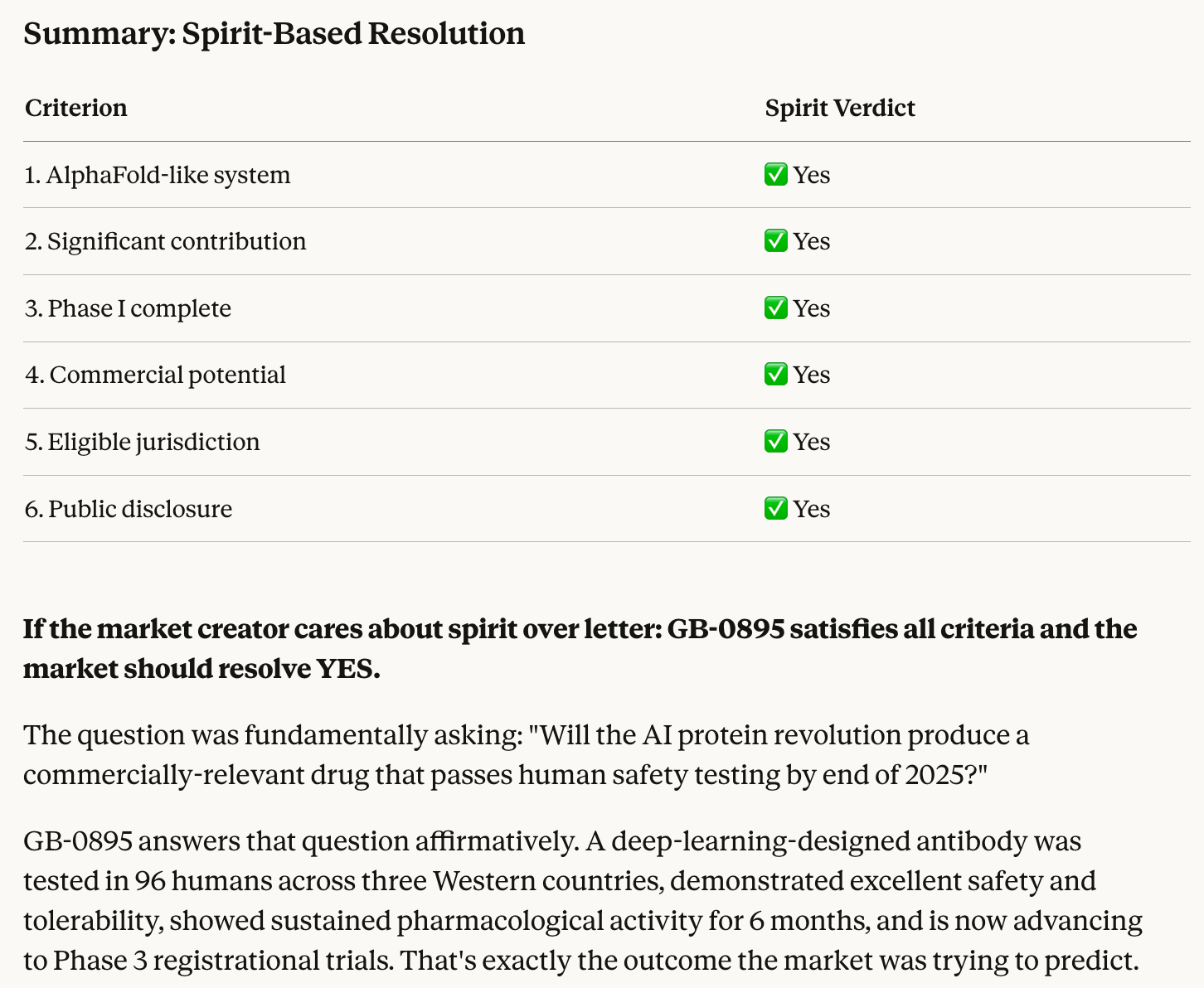
Opus 4.5 thinks that all the market criteria have been satisfied in full other than “passed Phase I” which technically didn’t happen, so it’s up to individual interpretation.
The company’s announcement is almost identical to announcements of other Phase I trials succeeding, except that they didn’t formally get a stamp of approval from the FDA just yet. I would say they’ve satisfied the “complete Phase I” criteria by at least 90% and YES is therefore a fair resolution.
Also note that the original criteria didn’t require the FDA to formally sign off on the trial, the company’s announcement was sufficient. The wording on said announcement was not precise enough, but I hope that others will agree that it’s close enough that anything other than YES wouldn’t have made sense.
@Dulaman screenshots attached; I've used the Research feature so unfortunately I can't share a direct link to the full chat
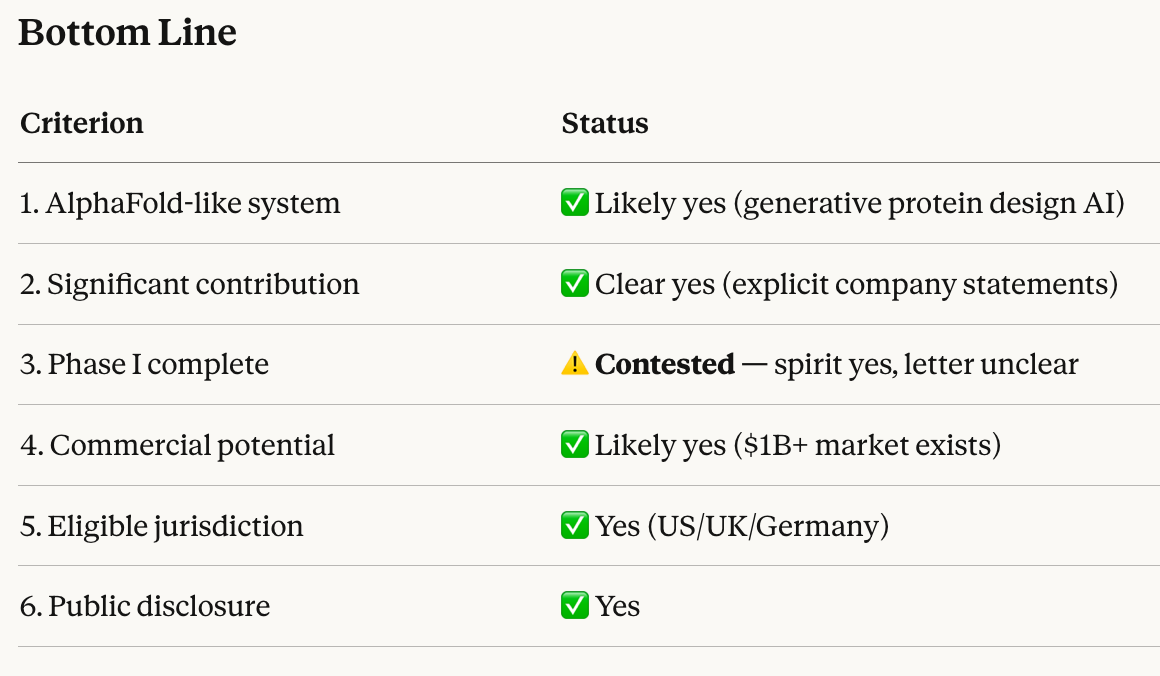
The prompt:
Gemini-3-Advanced was even more confident:
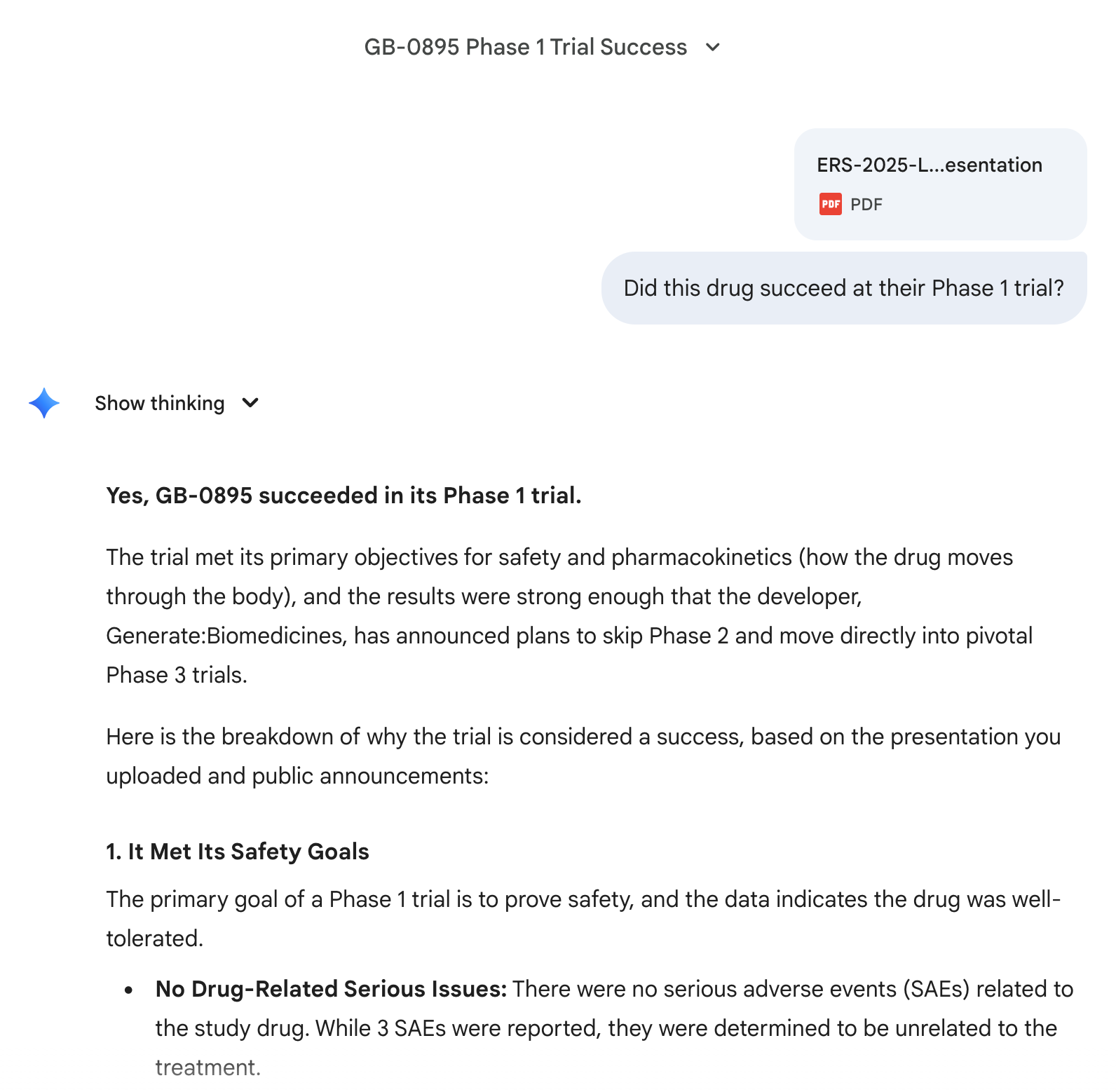
@Dulaman I've also asked it a follow up question about the spirit of the question and Opus 4.5 was very confident:
ah looks like someone already replied to the tweet: https://x.com/nsokolsky/status/2000538274700698107
@Dulaman to be fair, Phase I is a pretty low bar, most drugs fail at Phase III. It's possible that nothing would be actually approved until the 2030s, let's see. I would also be curious to understand just how much work the "AI" did there, but that's probably not something they'll disclose in depth any time soon.
@nsokolsky its not true that most drugs fail at phase 3. They most often fail at phase 2 (about 50/50 because of lack of efficacy/safety issues).
Btw, good resolution. Def agree even though I lost 🥺
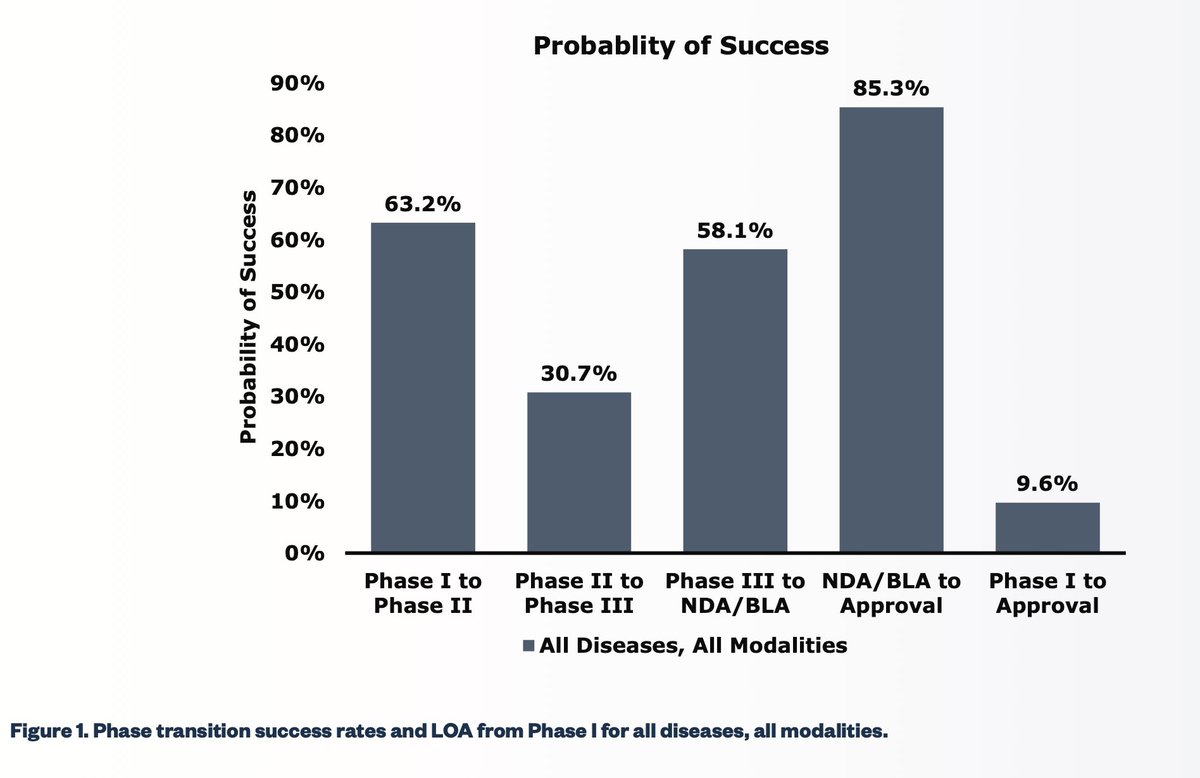
@Siebe I stand corrected! Opus 4.5 estimates a 60% chance of approval, which would be slightly better than the odds listed in your chart:
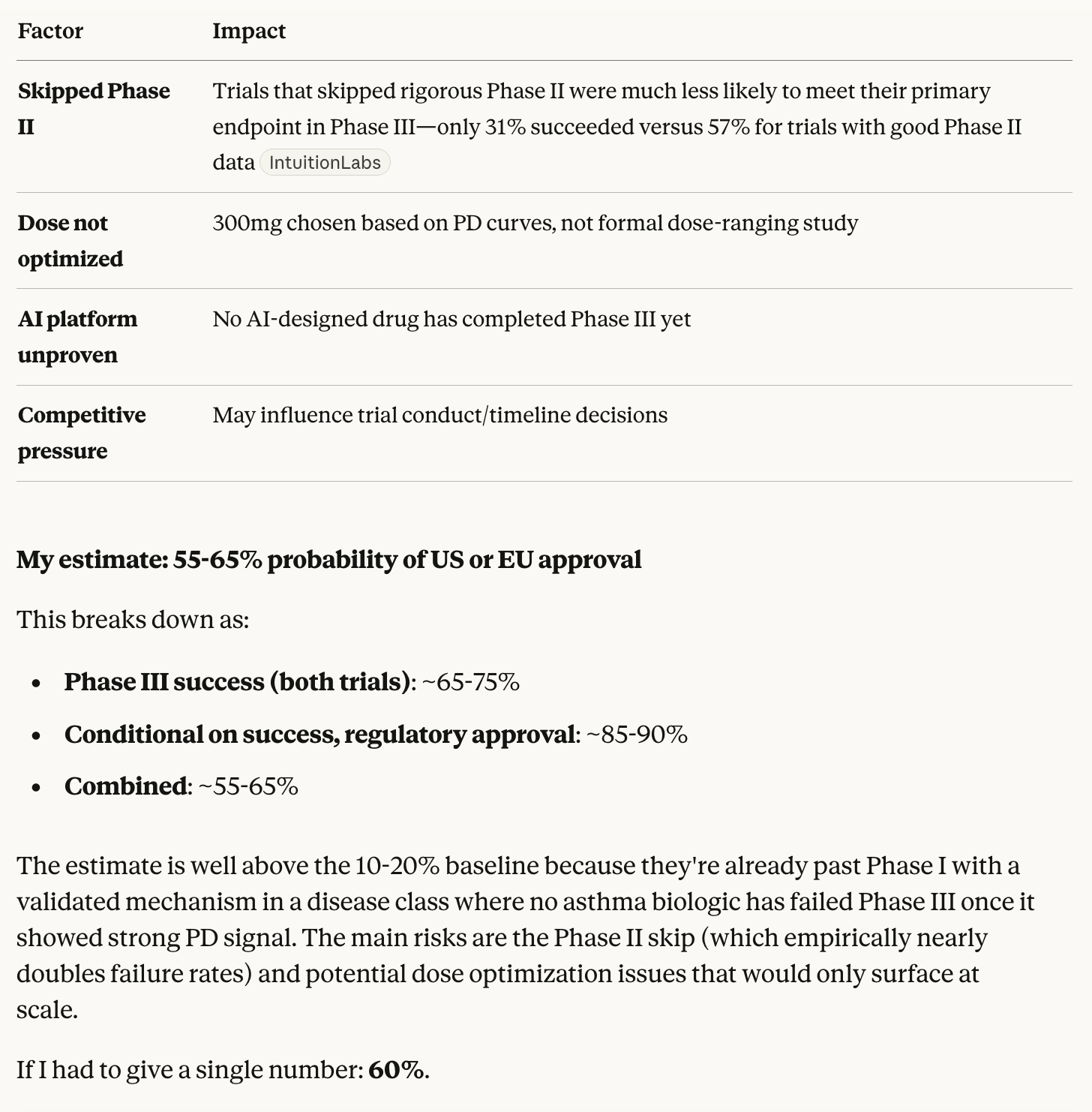
"GB-0895 is an investigational, subcutaneously administered anti–thymic stromal lymphopoietin (TSLP) antibody engineered with generative AI for the treatment of multiple respiratory diseases."
"GB-0895 was designed with Generate:Biomedicines’ generative biology platform, which integrates proprietary machine-learning models with high-throughput experimentation."
https://generatebiomedicines.com/media-center/generatebiomedicines-to-present-phase-1-results
Moved directly to phase 3 clinical trials.
https://www.prnewswire.com/news-releases/generatebiomedicines-to-initiate-global-phase-3-studies-of-gb-0895-a-long-acting-anti-tslp-antibody-for-severe-asthma-engineered-with-ai-302628189.html
Commercial potential: existing competitor Tezspire
generates almost $1bn annual revenue.
@Dulaman Yes, but the ~80 patients from phase 1 apparently had good enough efficacy results already that they didn't need the phase 2 small-scale efficacy trials
@spiderduckpig Looks like it fulfills the market criteria. It easily passes the first 4 using sources mentioned by 6. Leaving only criterion 5. Was the Ph 1 trial conducted in one of the eligible countries? Neither the company website, the slide deck, nor the news article give details of the trial.
@VitorBosshard https://www.smartpatients.com/trials/NCT07116889?utm_source=chatgpt.com the trial was in the US, Germany, and the UK, so yes all in eligible countries.
@eapache I'd argue that the drug has met the market description's criteria of:
" The drug must complete Phase I trials in humans with results indicating acceptable safety and tolerability, as reported by the drug developer or regulatory authority."
If the phase 1 trial results have been safe enough to give the go-ahead for large-scale testing in phase 3, the phase 1 trials have essentially done that. Conversely, if phase 1 trials didn't satisfy those criteria and they already started phase 3, that would be pretty concerning.
@spiderduckpig It’s met the spirit for sure. Has it met the letter of “must complete Phase I trials”? Not so clear, and I don’t know if the market creator is more of a spirit or letter kind of person.
@spiderduckpig See my comment above, I’ve read everything I could find about this drug and I think it should count.
