Will Vaxart’s oral COVID vaccine candidate show at least 75% efficacy in a human challenge trial?
12
Ṁ1kṀ2.8kresolved Jan 29
Resolved
NO1H
6H
1D
1W
1M
ALL
Background: Vaxart is planning to run a human challenge trial using the Omicron strain of COVID to test its oral vaccine candidate. See: https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-agreement-hvivo-develop-worlds-first-human
Resolves YES if the reported efficacy against infection is 75% or more, NO if under 75% efficacy, and N/A if the vaccine trial winds up not happening, or if efficacy against infection isn’t used as a clinical endpoint (although that seems unlikely).
This question is managed and resolved by Manifold.
Market context
Get  1,000 to start trading!
1,000 to start trading!
🏅 Top traders
| # | Trader | Total profit |
|---|---|---|
| 1 | Ṁ806 | |
| 2 | Ṁ13 | |
| 3 | Ṁ8 | |
| 4 | Ṁ2 | |
| 5 | Ṁ1 |
Sort by:
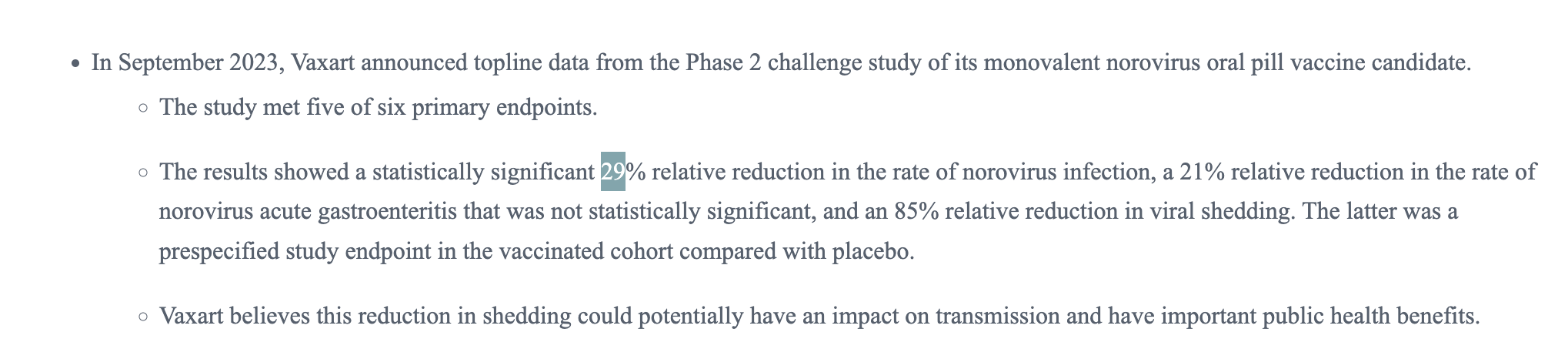
looks like it's gonna resolve NO
https://investors.vaxart.com/news-releases/news-release-details/vaxart-provides-business-update-and-reports-third-quarter-2023
People are also trading
Related questions
Will the VLA15 Lyme Disease vaccine "pass" its phase 3 human trial?
62% chance
Will the M72/AS01E tuberculosis vaccine candidate have an efficacy of 50% or higher in the phase 3 trial?
59% chance
If a human challenge trial for a hepatitis C vaccine is completed by the end of 2024, will a hep C vaccine be approved by the end of 2027?
53% chance
If a human challenge trial for a hepatitis C vaccine is NOT completed by the end of 2024, will a hep C vaccine be approved by the end of 2027?
46% chance
Will there be a successful human trial of a vaccine for rheumatoid arthritis by the end of 2028?
20% chance