
From https://metaculus.com/questions/15835/mifepristone-fda-approval-suspended/
According to the US Food and Drug Administration (FDA):
The FDA first approved Mifeprex (mifepristone) in September 2000 for medical termination of pregnancy through seven weeks gestation and this was extended to ten weeks gestation in 2016. FDA approved a generic version of Mifeprex, Mifepristone Tablets, 200 mg, in April 2019.
During the coronavirus pandemic, the FDA temporarily granted approval for patients to receive mifepristone by mail. This change was later made permanent.
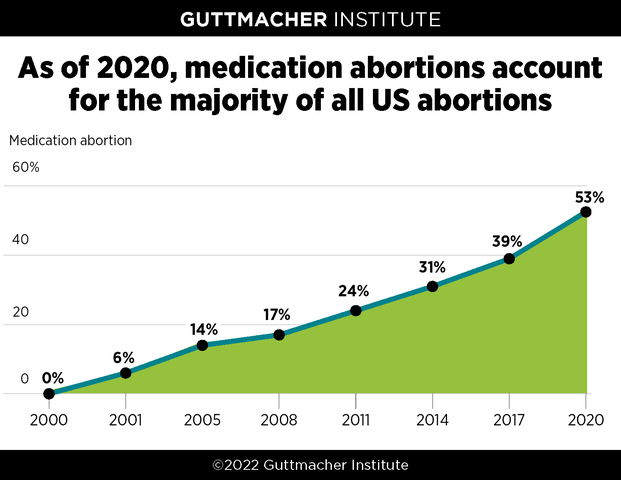
*[Guttmacher Institute, February 2022](https://www.guttmacher.org/article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions)*
On April 7, 2023, the Northern Texas US district court issued an opinion in the case Alliance for Hippocratic Medicine v. US Food and Drug Administration which issued a stay of enforcement of the FDA's approval of mifepristone. The opinion stayed the enforcement of its own opinion for seven days, allowing time for the federal government to seek emergency relief from the Fifth Circuit Court of Appeals.
Shortly after on the same day, the Eastern Washington US district court issued an opinion in the case State of Washington v. US Food and Drug Administration which enjoined the FDA from
altering the status quo and rights as it relates to the availability of Mifepristone under the current operative January 2023 Risk Evaluation and Mitigation Strategy under 21 U.S.C. § 355-1 in Plaintiff States.
The conflicting orders are expected to require resolution by higher courts.
🏅 Top traders
| # | Name | Total profit |
|---|---|---|
| 1 | Ṁ90 | |
| 2 | Ṁ25 | |
| 3 | Ṁ23 | |
| 4 | Ṁ10 | |
| 5 | Ṁ2 |